동향분석
Home > 뉴스브리핑센터 > 동향분석| 한약재 스크리닝을 통한 코로나바이러스의 증식 억제제 조사 및 억제 기전 규명 (아주대 의대 김경민 교수팀, 2007) |
|
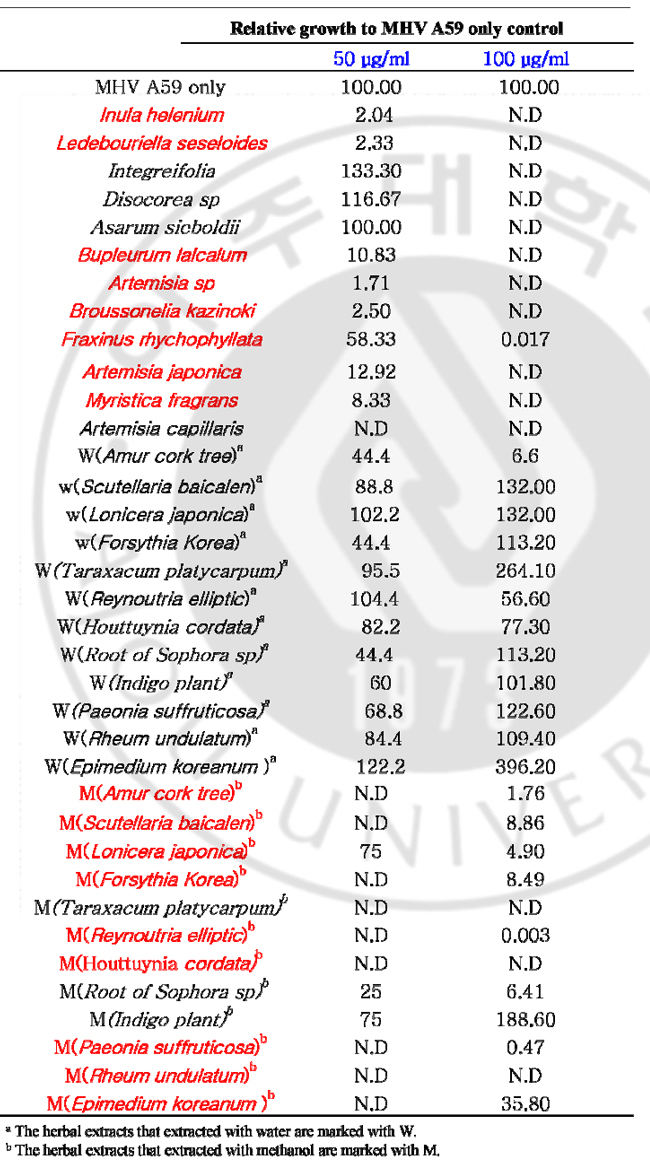
한약재 스크리닝을 통한 코로나바이러스의 증식 억제제 조사 및 억제 기전 규명 신현수, 김경민 (아주대학교 대학원 의학과) 국문 요약 목적: 신 변종 코로나바이러스인 SARS 코로나바이러스의 발생으로 SARS 코로나바이러스 감염의 치료 및 예방의 중요성이 부각되고 있다. 급성 호흡기 증후군을 유발하는 SARS 코로나바이러스의 진원지가 중국으로 한국과 지리적으로 인접해 있기 때문에 중국과 교류가 활발한 시점에서 농산물이나 여행자를 통해 SARS 코로나바이러스가 한국에 유입될 가능성이 아주 높다. 따라서 코로나바이러스의 증식을 억제하는 한약재 추출물을 스크리닝 한 후 이 약재들의 바이러스 억제 작용 및 억제 기전을 밝히고자 하며, 감염된 동물에게서 효과가 나타나면 축산업계에 손실을 줄일 수 있을 뿐 아니라 SARS 코로나바이러스 치료제로써 이용될 수 있을 것이다. 한약재 추출물이 코로나바이러스의 증식을 억제하는지를 조사한 후 이 약재들의 바이러스 억제 작용 및 억제 기전을 밝히고자 한다. 재료 및 방법: Delayed brain tumor cell (DBT) 세포와 mouse hepatitis virus (MHV) A59를 wild type 바이러스로써 사용하였다. 약재가 바이러스의 복제에 영향을 미치는지를 알아보기 위해서 DBT 세포에 바이러스와 약재를 동시에 감염시켜서 플라크 측정법과 Northern blot 을 시행하였다. 다음으로는 약재가 바이러스의 흡착에 영향을 미치는지의 여부를 관찰하기 위해서 우선은 DBT 세포에 바이러스를 흡착시킨 후 약재를 처리하여 플라크 측정법과 Northern blot 을 시행하였다. 그리고 다양한 농도의 약재가 바이러스에게 미치는 영향을 Northern blot 및 플라크 측정법을 통해 조사하였다. 약재가 세포 자체에 독성을 미치는지를 조사하기 위해서 MTT assay를 실행하였다. 결과: 40종류의 약재를 스크리닝 한 결과 약 12개의 약재가 바이러스의 증식을 억제하는 것으로 나타났다. 이 중에는 바이러스의 RNA 합성 및 복제를 억제하는 약재도 있었고, 일부는 특히 바이러스의 흡착 단계를 억제하는 약재도 있었다. 하지만 약재 자체가 세포에는 영향을 미치지 않는지를 보기 위한 MTT 실험 결과 12개의 약재들 중에 세포에 영향을 미치는 약재가 있었다. 현재 애엽, 진피, 어성초, 호장근이 세포에 영향을 거의 미치지 않으면서 바이러스의 RNA 복제, 혹은 흡착을 억제하는 약재로 밝혀졌다. 결론: 일부 약재들은 세포에 거의 영향을 미치지 않으면서 바이러스의 RNA 복제, 혹은 흡착을 억제하는 것으로 확인하였다. 키워드: 코로나바이러스 복제, 한약재 Abstract Coronavirus Replication Inhibition by Herbal Extracts Coronaviruses are plus-stranded RNA viruses that infect human and large variety of animals, including pig, cattle, dog, rat, rabbit, turkey, and bird. Human coronavirus has been known to cause common colds and perhaps gastroenteritis. Animal coronaviruses cause gastroenteritis, hepatitis, and a syndrome similar to multiple sclerosis of humans. The resent severe acute respiratory syndrome (SARS) outbreak has stirred out global, which turned out to be a novel zoonotic coronavirus. SARS is an infectious disease with a high potential for transmission by close contact. In search for the active viral compound to treat coronavirus infections including SARS coronavirus, I screened several herbal extracts from natural compounds affecting replication of mouse hepatitis virus (MHV), the prototype coronavirus, infected cells. The reduced virus production was analyzed by plaque assay. The replication inhibition of MHV by herbal extracts in terms of RNA replication was explored through the analysis of the intracellular viral RNAs. Plaque assay results showed that some of the herbal extracts inhibited coronavirus replication. Northern blot analyses showed that the intracellular viral RNA expressions were delayed by the herbal extracts. At present, several herbal extracts were identified that are effective for MHV reduction in cell culture system: Inula helenium (목향); Ledebouriella seseloides (방풍); Bupleururn lalcalum (시호); Artemisia sp (애엽); Artemisia japonica (청호); Myristica fragrans (초과); Amur cork tree (황백); Scutellaria baicalen (황금); Lonicera japonica (금은화); Forsythia Korea (연교); Reynoutria elliptic (호장근); Houttuynia cordata (어성초); Paeonia suffruticosa (목단피); Rheum undulatum (대황); Epimedium koreanum (음양곽). Keywords: coronavirus replication, herbal extracts, antiviral compounds I. INTRODUCTION A. Overview Coronaviruses, belonging to the coronaviridae family, are plus-stranded, positive-sense RNA viruses. Coronavirus virions are spherical, enveloped virus particles with surface projections, which are 80-160nm in diameter (Sturman et al., 1980). Coronaviruses infect human and large variety of animals, including pig, cattle, dog, rat, rabbit, turkey, and bird (Wege et al., 1982; Lai, 1990). Most coronaviruses infect only one or several closely related species, and viral replication is often restricted to epithelial cells of the respiratory or enteric tracts and macrophage. In human, coronavirus has been known to cause common colds and perhaps gastroenteritis (Lai, 1990). Coronaviruses exhibit strong species and tissue specificity (McIntosh, K., 1974). They have the largest genome of all RNA viruses and replicate by a unique mechanism which results in a high frequency of recombination. Virions mature by budding at the ER or cis Golgi (Tooze et al., 1974; Wege et al., 1982), utilizing the intracellular membrane as an envelope, and induce the cell-fusion of susceptible cells. The prototypical coronaviruses include avian infectious bronchitis virus (IBV), mouse hepatitis virus (MHV), porcine transmissible gastroenteritis virus (TGEV), bovine coronavirus (BCV), human coronavirus (HCV), feline infectious peritonitis virus (FIPV), turkey coronavirus (TCV), rabbit coronavirus (RCV), and several viruses of other animal species. B. Virion structure of coronavirus All coronavirus virion particles contain four or five structural protein; spike (S), membrane (M), hemagglutinin-esterase (HE, nucleocapsid (N), and small membrane (E) proteins. The S protein constitutes the spike or peplomers on the virion envelope. Its molecular weight is 180kD and it is cleaved into two proteins, N-terminal S1 and Cterminal S2, of 90kD each. It contains an N-terminal signal sequence, a C-terminal hydrophobic, transmembrane domain (Sturman et al., 1985), and a large number of potential N-linked glycosylation sites (Stern and sefton; 1982). The attachment of coronavirus to viral receptor on target cells is mediated by the S protein (Collins et al., 1992). S-induced cell-cell fusion dose not require the cleavage of S protein because it is known that S protein of TGEV is not cleaved but can induce syncytia. Also prevention of cleavage dose not result in loss of fusion activity. Since fusion of incoming virion with cell membrane is sometimes affected to a certain extent by change in pH, virioncell fusion occurs at the cell surface or within endosomes prior to their acidification in lysosome (Cavanagh, 1995). It is responsible for the induction of neutralizing antibody and cell-mediated immunity (Sturman and Holmes, 1983). This spike protein is a major determinant of cell tropism and pathgenicity. The M protein is a triple spanning membrane glycoprotein, with large cytoplasmic C- terminal domain, and small N-terminal domain which is glycosylated by N-linkage in IBV (Stern and Sefton 1982) and TGEV (Laude et al., 1987), or by O-linkage in MHV (Niemann et al., 1984). Since Golgi retention signal for M protein resides on its first transmembrane domain (Swift and Machamer, 1991), it accumulated in the late Golgi structure when expressed alone (Klumperman et al., 1994). Because intracellular distribution of M protein correlate with budding site, ER or cis-Golgi (Tooze et al., 1984), it is believed to be a key determinant of coronavirus assembly. But it was suggested that M protein might not be the sole determinant because it is transported beyond the budding site (Klumperman et al., 1994). The 65kD HE is an optional glycoprotein because it is present in BCV, TCV, HCV, and same strains of MHV, but absent in IBV and TGEV (Lai ,1990). This protein is glycosylated by N-linked carbohydrates (Yokomori et al., 1989) and constitutes the smaller spike of virion. BCV HE protein has hemagglutinating and esterase activity (Vlasak et al., 1989). And some strains of MHV have esterase activity (Yokomori et al., 1989). The esterase activity represents the receptor destroying activity in influenza C virus (Vlasak et al., 1987). The HE protein is dispensible, but may have an unknown luxury function for the virus (Lai, 1990). The N proteins are phosphoprotein of 50-60kD (Stohlman and Lai, 1979) and bind to virion RNA (Sturman et al., 1990). Phosphorylation of N protein occurs by posttranslational modification (Stohlman and Lai, 1979) and it is speculated that this phosphorylation may govern to tighten the association between N protein and viral RNA. The N protein also bind to other N and M proteins. A helical nucleocapsid of 6-8nm in diameter is released from envelope by mild detergent treatment (Sturman et al., 1980), which consists of genomic RNA and N protein. N proptein may have not only a structural role but also a regulatory role in viral replication because anti-N antibody inhibits MHV RNA synthesis in vitro. C. Genome structure of coronavirus Coronvirus contains a single-stranded, positive sense RNA genome, approximately 27-32kb in size (Lee et al., 1991). The genomic RNA contains a 5’ cap structure and 3’ poly A tail and is infectious upon transfection of naked RNA into a susceptible cell line. The MHV genome contains eight or nine genes. Sequence analysis of the virion genomic RNA revealed the presence of at least 10 open reading frames (ORFs). There ORFs encode various structural and nonstructural proteins of coronaviruses. These are several overlapping ORFs within a gene. For example, genes 1 and 5 of MHV have two ORF and genes 1and 3 of IBV have two and three ORFs, respectively. The 5’ most gene, gene 1, is from 18kb long in IBV to 22kb long in MHV and encode viral RNA polymerase and protease that are necessary for viral RNA synthesis (Lee et al., 1991). In MHV and BCV, ns2 gene product of 30kD and gene 2-1 product of HE protein, 65kD glycoprotein, are synthesized. Because an MHV mutant with the majority of gene 2 deletion replicate well in tissue culture, the gene 2 and 2-1 product are not essential for MHV replication at least in tissue (Schwart et al., 1991). The ns4 and ns5a proteins are not necessary for MHV replication, because mRNA 4 is not detected and 5a ORF is deleted in MHV-S infected cells. D. Coronavirus replication Coronaviruses have very restricted host range and tissue specificity. For example, MHV only infects cells of gastrointestinal tracts, reticuloendothelial system, and nervous system. This host and tissue specificity may be controlled at the cellular receptor level. The presence and the absence of this membrane receptor protein correlates with the sensitivity or resistance of mouse strains. The receptor molecule on the surface of target cells appears to provide the entry points for viral infection where the S protein of coronavirus interacts with the receptors. After binding, the virus penetrates into the host cell by cell fusion or by viropexis and un-coats through endosomal fusion. Viral replication cycle takes place in the cytoplasm after the release of viral genome into the cell. The first event threreafter is the synthesis of a virus encoded RNA-dependent-RNA polymerase. Since this enzyme is not carried in the virus particle, it has to be synthesized de novo from the incoming genomic RNA. The replication of coronavirus RNA requires continued protein synthesis from the very beginning of viral infection. This virus-encoded RNA-dependent-RNA polymerase transcribes the incoming viral genomic RNA into full-length and sub-genomic negative stranded RNA, which then serve as the templates for the synthesis of genomic and subgenomic mRNAs. The majority of viral RNAs are poly A containing mRNAs which are separated into 6-8 species. They are named mRNAs 1 to 7 in order of decreasing size. The largest mRNA 1 is identical to the genomic RNA of the virion. These mRNA have a nested set of RNA structure. All sequences of the mRNAs start from the 3’ end of the genome and extend for various distances in the 5’ end direction. Therefore all the mRNAs have overlapping sequences in the 3’ end and are polycistronic in their structure except for the smallest mRNA. Thus, all mRNA have a 3’-coterminal nested set structure. But only the 5’ portion of each mRNA is translatable. Therefore these mRNAs are functionally monocistronic. The coronavirus mRNA synthesis is detected a few hours after virus infection in most virus cell systems. The MHV leader is roughly 72-77 nucleotides long while that of IBV is roughly 60 nucleotides. A stretch of 7-18 nucleotides between the 3’ end of the leader RNA and intergenic sequence is homologous. Those homologous region is assumed to be the point of leader RNA binding and priming. Within this homologous region, seven nucleotide are conserved among most coronaviruses, except IBV which has the UCUAAAC sequence instead. These conserved sequence vary slightly among different intergenic sequences, and these conserved regions are probably the consensus sequences recognized by RNA polymerase for mRNA transcription. Several additional mRNA are detected in some MHVs. For example, a mRNA 2-1 corresponds to functional HE gene but is transcribed only in a few strains. It is transcribed only in JHM strains containing two UCUAA repeat in the leader sequence at the 5’ end of genome, but not by JHM strain with three UCUAA repeat (Makino et al., 1989). The viral mRNAs are then used for the synthesis of structural and nonstructural proteins. Some of nsp participate in viral RNA synthesis and others may be involved in virus assembly or shut off of host macromolecular synthesis. The genomic sized RNA has two populations; one is a mRNA 1, which is used for the translation of the 5’ most ORF, and the other is the RNA that is packaged into virion. In BCV infected cell, the relative ratio of the genomic to sub-genomic RNAs increase later in infection, suggesting that mRNA synthesis shift to genomic RNA synthesis. Most of the genomicsized RNA is associated with nucleocapsids. Accumulation of viral structural proteins is required for the assembly of virus particle and may signal the switch from synthesizing mRNAs to making genomic RNA that get packaged into the virion. E. Purpose of research The resent severe acute respiratory syndrome (SARS) outbreak has stirred out global, which turned out to be a novel zoonotic coronavirus. SARS is an infectious disease with a high potential for transmission by close contact. Since the epidemic, a lot of effort has been put into antiviral research to find effective compounds against SARS coronavirus. Glycyrrhizin (J. Cinatl, M. Michaelis et al., 2003), niclosamide (C.J Wu et al. 2004), nelfinavir (N. Yamamoto et al. 2004) were reported to have antiviral effects against SARS coronavirus. In search for the active antiviral compounds to treat infectious caused by coronaviruses including SARS coronavirus, I screened 40 herbal extracts from natural compounds in mouse hepatitis virus (MHV), the prototype coronavirus, infected cells. Ⅱ. MATERIAL AND METHOD A. Cell culture and Virus Delayed brain tumor (DBT) cells were maintained in MEM (minimum essential medium, Gibco BRL, Grand Island, NY) medium supplemented 8% bovine serum, 10% tryptose phosphate broth (TPB), penicillin and streptomycin serum. Mouse hepatitis virus (A59) was used as a wild type virus. B. Herbal extracts Following herbal extracts were tested: Bupleururn lalcalum; Artemisia sp; Artemisia japonica; Myristica fragrans; Amur cork tree; Scutellaria baicalensis; Lonicera japonica; Forsythia koreana; Reynoutria elliptica; Houttuynia cordata; Paeonia suffruticosa; Rheum undulatum; Epimedium koreanum,; Inula helenium; Ledebouriella seseloides; Seed of Brassica juncea var; Asarum sieboldii; Broussonelia kazinoki; Fraxinus rhychophyllata; Roots of sophora sp; Indigo plant; Astragalus membraneaceus. C. Infection of coronavirus DBT cells were seeded on a 12-well plate. The DBT cells were infected with MHV A59 at 2 multiplicity of infection (MOI) at 37 °C, treated with herbal extracts at the same time or herbal extracts were added to MHV pre-adsorbed-DBT cells. After 1hrs of incubation, fresh medium (88% MEM, 10% TPB 2% FBS, penicillin and streptomycin) was added. After 12hrs incubation, viruses were harvested. D. Screening of herbal extracts The DBT cells were infected with MHV A59 at 2 multiplicity of infection (MOI) at 37 °C, treated with herbal extracts at the same time and herbal extracts were added to MHV pre-adsorbed-DBT cells. After 12hrs incubation, viruses were harvested and released viruses were titrated by plaque assay on DBT cells. 48hrs after infection, cells were stained with neutral red. E. Preparation of cytoplasmic RNA The intracellular virus-specific RNA was extracted as described previously (Makino et al., 1984b). After infection, DBT cells were lysed in 200μℓ NTE(100mM NaCl, 10mM Tris [pH7.5], 1mM EDTA)-0.5%Nonidet P-40. The clarified lysate was treated with proteinase K (100μg/mL) and proteinase K Buffer (0.2M Tris, 25mM EDTA, 0.3M NaCl, 2% SDS) treatment, and extracted with phenol and chloroform. F. Northern blotting For each sample, 2μg of cytoplasmic RNA was denatured and electrophoresed through a 1% agarose gel containing formaldehyde and then transferred to nylon membrane. RNA on the membrane was hybridized to 32P-labeled probe specific for the 3’ end of MHV sequence. G. MTT assay An MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] cell proliferation assay was used to determine potential cytotoxicity of the compound. 1 x 10⁴DBT cells per well were seeded on a 96-well plate. 1 μg, 10 μg, 50 μg, and 100 μg of DMSO dissolved compounds were added to DBT cells. After 8 hrs of incubation, 10 μl of MTT was added and cells were incubated for another 3.5 h. The plates were swirled and measured with a microtiter plate reader at 570nm. III. RESULT A. Forty herbal extracts were screened for antiviral activity against mouse hepatitis virus Forty herbal extracts were screened for antiviral activity against mouse hepatitis virus (MHV), the prototype coronavirus (Table 1). The DBT cells were infected with MHV A59 at 2 multiplicity of infection (MOI) at 37 °C, treated with herbal extracts (50μg, 100μg) at the same time. After 12 hr incubation, viruses were harvested and released viruses were titrated by plaque assay on DBT cells. Plaque assay results showed that following herbal extracts inhibited coronavirus replication: Amur cork tree; Scutellaria baicalen; Lonicera japonic; Forsythia Korea; Reynoutria elliptic; Houttuynia cordata; Paeonia suffruticosa; Rheum undulatum; Epimedium koreanum were extracted methanol compounds. Inula helenium; Ledebouriella seseloides; Bupleururn lalcalum; Artemisia sp; Broussonelia kazinoki; Fraxinus rhychophyllata; Artemisia japonica; Myristica fragrans. This experiment was repeated two times, and representative results are shown in Table 1. Table 1. Plaque assay of MHV A59 on DBT cells at 37℃ for the screening of herbal extracts
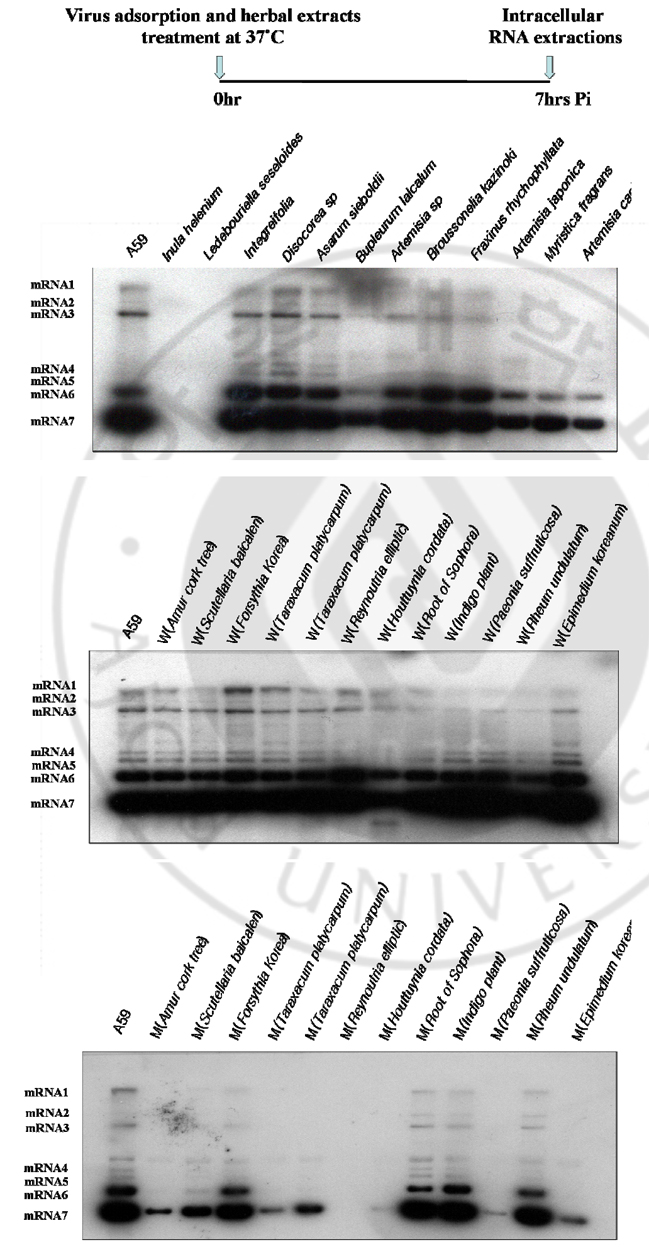
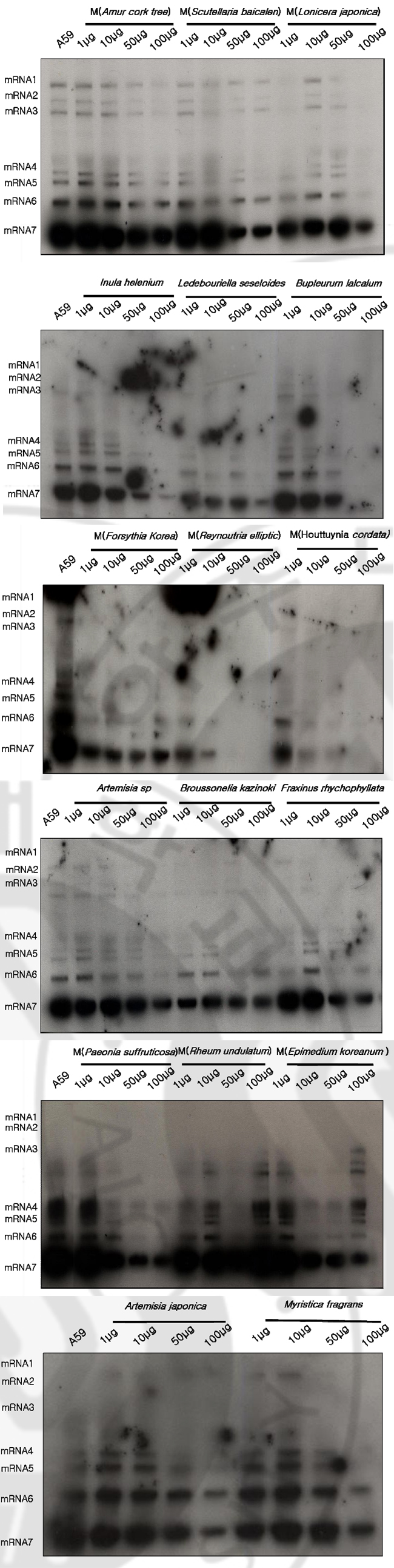
B. Effects of herbal extracts on MHV RNA expression through MHV infectious cycle including virus entry and after virus adsorption At 7 hrs of infection, cytoplasmic RNA was extracted from murine DBT monolayers that had been infected with MHV A59 at 2 multiplicity of infection (MOI) and treated with herbal extracts (100μg) at the same time (Fig. 1). Cytoplasmic RNA (2μg) was denatured and electrophoresed on a 1% agarose gel containing formaldehyde, blotted to nylon membrane, and hybridized with random-primed 32Plabeled MHV-specific probes. Some herbal extracts are shown to be effective on MHV RNA reduction in cell culture system. Among those Amur cork tree; Scutellaria baicalen; Forsythia Korea; Reynoutria elliptic; Houttuynia cordata; Paeonia suffruticosa), and Epimedium koreanum are effective on inhibition of viral RNA synthesis. At 7 hrs of infection, cytoplasmic RNA was extracted from murine DBT monolayers that had been treated with herbal extracts (100μg) to MHV pre-adsorbed-DBT cells. (Fig. 2). Cytoplasmic RNA (2μg) was denatured and electrophoresed on a 1% agarose gel containing formaldehyde, blotted to nylon membrane, and hybridized with random-primed 32P-labeled MHV-specific probes. Some herbal extracts are shown to be effective on MHV RNA reductions in cell culture system. Among those, Reynoutria elliptic; Houttuynia cordata), and Epimedium koreanum are effective on inhibition of MHV entry.
Fig. 1. Northern blot analyses to detect MHV RNA expressions by herbal extracts on DBT cells DBT cells were infected with MHV A59 at 2 multiplicity of infection (MOI) and treated with herbal extracts (100μg) at the same time. Cytoplasmic RNA (2μg) was denatured and electrophoresed on a 1% agarose gel containing formaldehyde, blotted to nylon membrane, and hybridized with random-primed 32P-labeled MHVspecific probes.
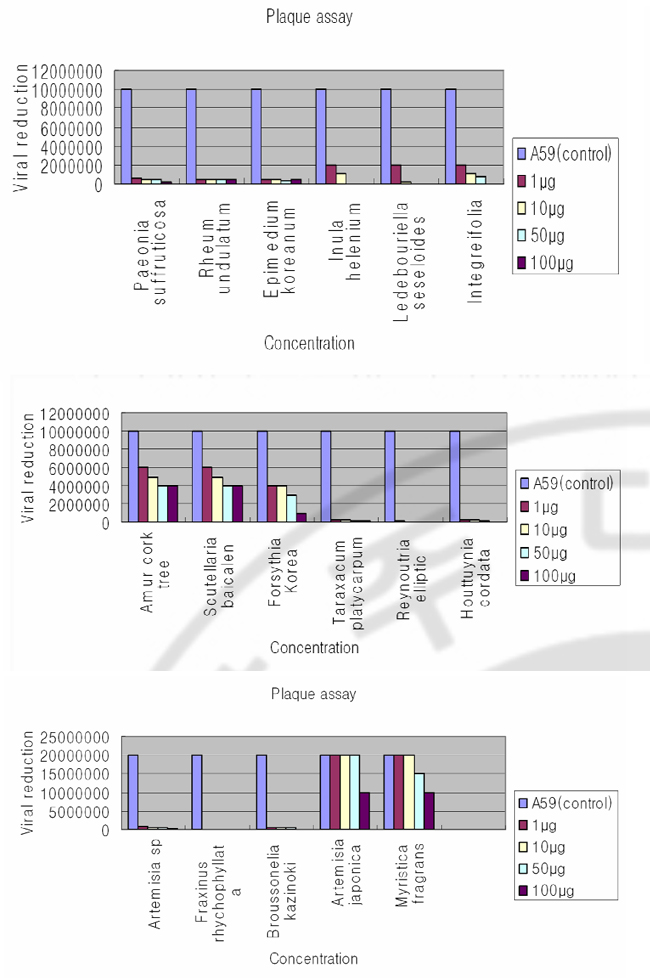
Fig. 2. Northern blot analyses to detect MHV RNA expressions by herbal extracts on DBT cells Herbal extracts (100μg) were added to MHV pre-adsorbed-DBT cells. Cytoplasmic RNA (2μg) was denatured and electrophoresed on a 1% agarose gel containing formaldehyde, blotted to nylon membrane, and hybridized with randomprimed 32P-labeled MHV-specific probes. C. Plaque assay of MHV A59 by varying concentrations of herbal extracts Following herbal extracts are shown to be effective on MHV RNA reductions in cell culture system: Inula helenium; Ledebouriella seseloides; Bupleururn lalcalum; Artemisia sp; Broussonelia kazinoki; Fraxinus rhychophyllata; Artemisia japonica; Myristica fragrans; Amur cork tree; Scutellaria baicalen; Lonicera japonica; Forsythia Korea; Reynoutria elliptic; Houttuynia cordata; Paeonia suffruticosa; Rheum undulatum; Epimedium koreanum). As a further study, I assayed these herbal extracts at varying concentrations by plaque assay (Fig. 3). DBT cells were infected with MHV A59 at 2 MOI and treated with herbal extracts (1μg, 10μg, 50μg, 100μg) at the same time. After 12 hr incubation, viruses were harvested and released viruses were titrated by plaque assay on DBT cells. Plaque assay results show that following herbal extracts at varying concentrations inhibited coronavirus replication: Inula helenium; Ledebouriella seseloides; Bupleururn lalcalum; Artemisia sp; Broussonelia kazinoki; Fraxinus rhychophyllata; Forsythia Korea; Reynoutria elliptic; Houttuynia cordata; Paeonia suffruticosa; Rheum undulatum; Epimedium koreanum.
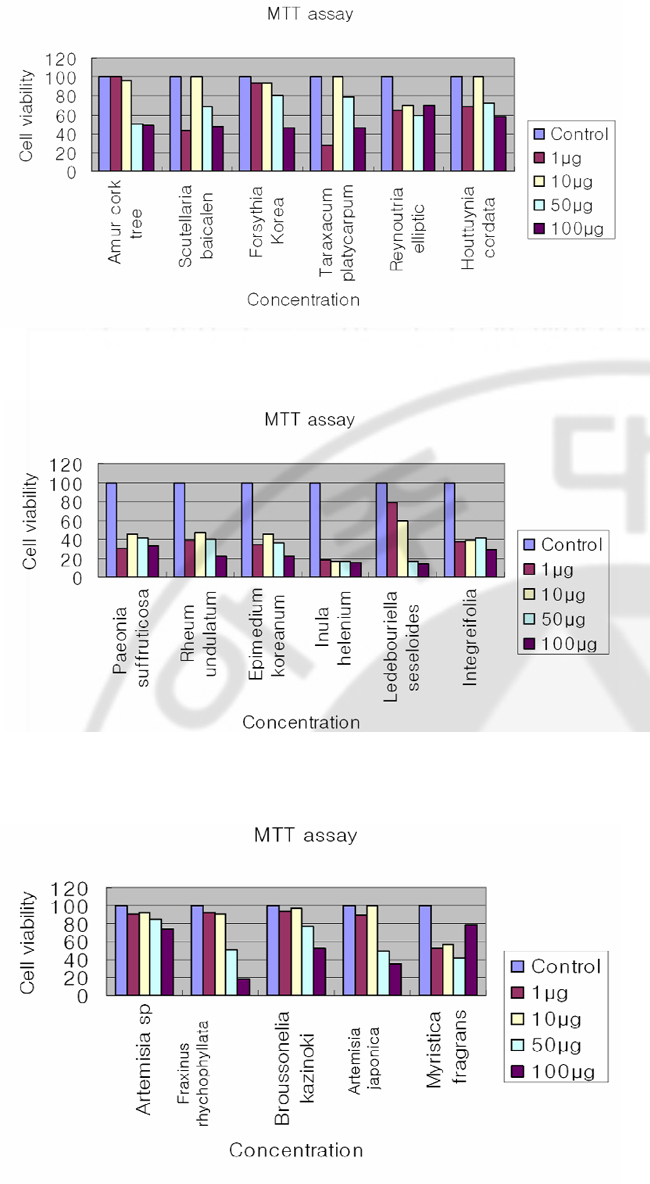
Fig. 3. Plaque assay of MHV A59 on DBT cells by herbal extracts The DBT cells were infected with MHV A59 at 2 multiplicity of infection (MOI) and treated with herbal extracts (1μg, 10μg, 50μg, 100μg) at the same time. After 12 hr incubation, viruses were harvested and released viruses were titrated by plaque assay on DBT cel l s . 48 hr af t er infect ion, cel l s were s t ained wi th neut ral red. D. Inhibition of coronavirus without cytotoxicity Herbal extracts that are effective on MHV RNA reductions in cell culture system are: Inula helenium; Ledebouriella seseloides; Bupleururn lalcalum; Artemisia sp; Broussonelia kazinoki; Fraxinus rhychophyllata; Artemisia japonica; Myristica fragrans; Amur cork tree; Scutellaria baicalen; Lonicera japonica; Forsythia Korea; Reynoutria elliptli; Houttuynia cordata; Paeonia suffruticosa; Rheum undulatum), and Epimedium koreanum. I performed an MTT assay to exclude the possibility that the observed antiviral activity was due to compound-mediated cytotoxicity (Fig. 4). An MTT [3-(4,5-dimethythiazil-2yl)-2,5-diphenyl tetrazolium bromide] cell proliferation assay was used to determine potential cytotoxicity of the compound. 1ⅹ10⁴DBT cells per well were seeded on a 96-well plate and 1μg, 10μg, 50μg, and 100μg of DMSO dissolved compounds were added to DBT cells. After 8hrs incubation, 10μg of MTT was added and cells were incubated for another 3.5hr. The plates were measured with a microtiter plate reader at 570nm. The results indicate that following are effective on inhibition of MHV with minimal cytotoxicity: Artemisia sp; Fraxinus rhychophyllata; Reynoutria elliptic; Houttuynia cordata. Scutellaria baicalen, Lonicera japonica, and Ledebouriella seseloides, Fraxinus rhychophyllata, and Artemisia japonica show cytotoxicity at higher concentration, but show minor cytotoxicity at low concentration. Paeonia suffruticosa), Rheum undulatum), Epimedium koreanum), and Inula helenium, Ledebouriella seseloides, Bupleururn lalcalum show cytotoxicity even at lowest concentration (1μg/ml).
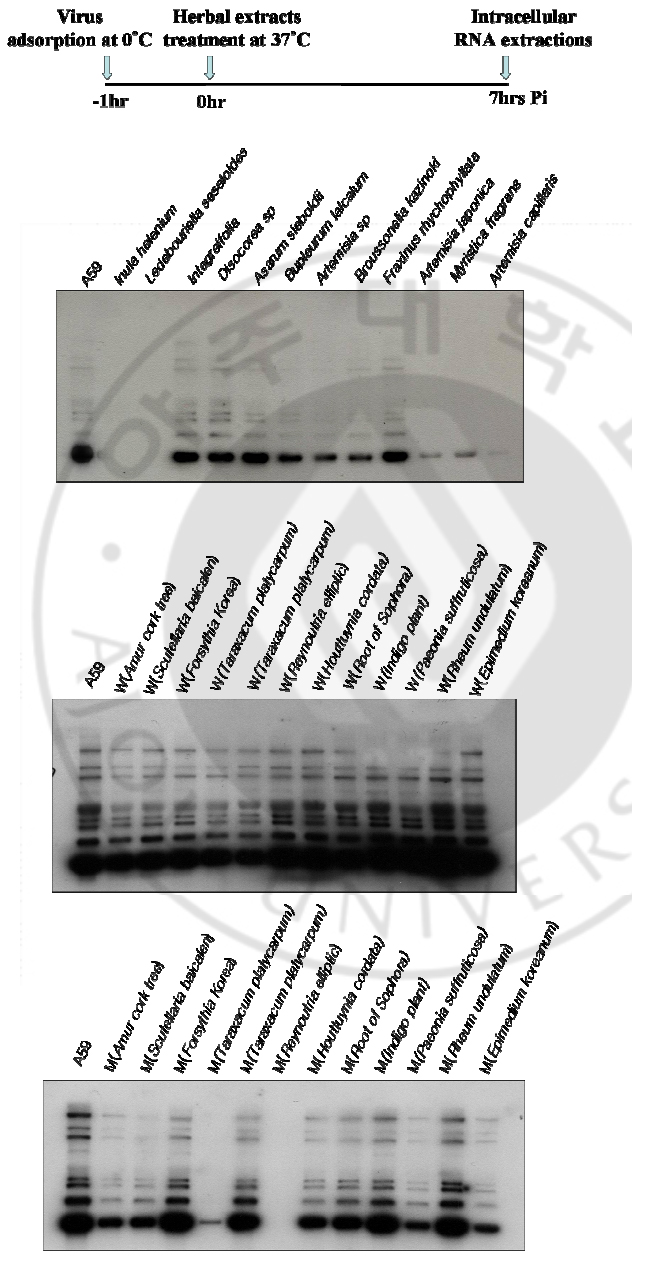
Fig. 4. MTT assay to determine the cell cytotoxicity of herbal extracts An MTT [3-(4,5-dimethythiazil-2yl)-2,5-diphenyl tetrazolium bromide] cell proliferation assay was used to determine potential cytotoxicity of the compound 1ⅹ10⁴DBT cells per well were seeded on a 96-well plate. 1μg, 10μg, 50μg, 100μg of DMSO dissolved compounds were added to DBT cells. After 8hrs incubation 10μg of MTT was added cells were incubated for another 3.5hr. The plates were swirled and measured with a microtiter plate reader at 570nm. E. Varying concentration herbal extracts on MHV RNA expression through MHV infectious cycle including virus entry Herbal extracts are effective on MHV RNA reductions in cell culture system: Inula helenium; Ledebouriella seseloides; Bupleururn lalcalum; Artemisia sp; Broussonelia kazinoki; Fraxinus rhychophyllata; Artemisia japonica; Myristica fragrans; Amur cork tree; Scutellaria baicalen; Lonicera japonica; Forsythia Korea; Reynoutria elliptli; Houttuynia cordata; Paeonia suffruticosa; Rheum undulatum), and Epimedium koreanum. Northern blot analyses to detect MHV RNA expression on DBT cell at varying concentration of herbal (Fig. 5). DBT cells were infected with MHV A59 at 2 (MOI) and treated with herbal extracts varying concentrations at the same time. The synthesis of viral RNA by herbal extract was analysed by Northern blotting. Increasing the concentration of the herbal extracts did suppress MHV RNA level: Inula helenium); Ledebouriella seseloides; Bupleururn lalcalum; Artemisia sp; Artemisia japonica); Myristica fragrans ; Forsythia Korea; Reynoutria ellipti; Houttuynia cordata; Paeonia suffruticosa).
Fig. 5. Northern blot analyses to detect MHV RNA expression on DBT cell at varying concentration of herbal extracts DBT cells were infected with MHV A59 at 2 multiplicity of infection (MOI) and treated with varying concentration herbal extracts at the same time. Cytoplasmic RNA (2μg) was denatured and electrophoresed on a 1% agarose gel containing formaldehyde, blotted to nylon membrane, and hybridized with random-primed 32P-labeled MHV-specific probes. IV. DISCUSSION Many herbal extracts have been reported to have strong antiviral activity and some of them have already been used to treat animal and people who suffer from viral infection (Hudson 1990; Venkateswaran 1987). Research interests for antiviral agent development was started after the Second World War in Europe and in 1952 the Boots drug company at Nottingham, England, examined the action of 288 plants against influenza A virus in embryonated eggs. They found that 12 of them suppressed virus amplification (Chantrill et al. 1952). During the last 25 years, there have been numerous broad-based screening program initiated in different parts of the globe to evaluate the antiviral activity of medicinal plants for in vitro and in vivo assays. Canadian researchers in the 1970s reported antiviral activities against herpes simplex virus, poliovirus type 1, coxsackievirus B5 and echovirus 7 from grape, apple, strawberry and other fruit juices (Kono-walchuk and Speirs 1976, 1978). Liquorice roots (감초) inhibited SARS-associated coronavirus replication (J Cinatl et al. 2003). In this study I report the in vitro antiviral activity of a few herbal extracts against MHV. Forty herbal extracts were screened and some were effective on reduction of MHV replication. Also, I assayed the antiviral effects of herbal extracts on the decrease of MHV RNA by Northern blot analyse. At 7hrs of infection, cytoplasmic RNA from murine DBT monolayers that had been treated with herbal extracts (100μM) at the of infection, was prepared (Fig. 1). Following Northern blot analyses show that the intracellular viral RNA expressions were delayed by methanol-extracted Amur cork tree), Scutellaria baicalen, Forsythia Korea, Reynoutria elliptic, Houttuynia cordata, Paeonia suffruticosa, and Epimedium koreanum, but were not delayed by waterextracted herbal compounds. As for the related studied, cytoplasmic RNA from murine DBT monolayers that had been treated with herbal extracts (100μM) to MHV pre-adsorbed-DBT cells was extracts (Fig. 2). At 7hrs of infections, herbal extracts are shown to be effective on MHV RNA reductions in cell culture system. Among those, MHV entry was inhibited by a methanol-extracted Reynoutria elliptic, Houttuynia cordata, Epimedium koreanum. Among Forty herbal extracts that have been screened, twelve extracts were found to have antiviral activity at the tested concentrations. As a further studied, I assayed the reduction of MHV RNA at varying concentration of herbal extracts by plaque assay (Fig. 3) and Northern blot analyses (Fig. 5). Northern blot result shown that increasing the concentration of the herbal extracts did suppress MHV RNA level. MTT assay was preformed to exclude the possibility that the observed antiviral activity was due to compound-mediated cytotoxicity (Fig. 4). The results indicate that a few are effective on inhibition of MHV without cytotoxicity: Artemisia sp; Fraxinus rhychophyllata; Reynoutria elliptic; Houttuynia cordata. In summary, the identification of several herbal extracts represent the first step toward the development of potential anti-coronaviral therapy. Several herbal extracts are broad spectrum of antiviral activity without detectable cytotoxicity in cell culture system. V. CONCLUSION In search for the active antiviral compounds to treat coronavirus infections including SARS coronavirus, I screened 40 herbal extracts from natural compounds in mouse hepatitis virus (MHV), infected cell. The reduced virus production was analyzed by plaque assay. The MHV RNA replication inhibition by herbal extract was explored through the analyses of the intracellular viral RNAs. Plaque assay results show that a few of the herbal extracts inhibited coronavirus replication. Northern blot analyses show that the intracellular viral RNA expressions were delayed by herbal extracts. At present, several herbal extracts have broad spectrum of antiviral activity without detectable cytotoxicity in cell culture system. REFERENCES 1. Boursnell MEG, Brown TDK, Fould IJ: Completion of the sequence of the genome of the coronavirus avian infectious bronchitis virus. J Gen virus 68:57-77, 1987. 2. Collins AR, Knobler RL, PowellH, Buchmeier MJ: Monoclonal antibodies to murine hepatitis virus-4 define the viral glycoprotein resposible for attachment and cell-cell fusion. Virology 119:358-371:1982. 3. Clnatl J Jr, Michaelis M, Hoever G, Preiser W, Doerr HW: Development of antiviral therapy for severe acute respiratory syndrome. Antiviral Res. Apr 26 2005. 4. De root RJ, Van Leen RW, Dalderup MJM: Stably expressed FIPV peplomer protein induce cell fusion and elicits neutralizing antibodies in mice. Virology 71;493-502:1989. 5. Eleouet J-F, Rasschaert D, Lambert P: Complete sequence of the polyproteinencoding gene1 of Transmissible gastroenteritis virus. Virology 206:817-822:1995. 6. Fosmire, J. A. Kim KH, and Makino S: Identification and charaterization of a coronavirus packaging signal. J.Virol 66:3522-3530:1992. 7. Kim KH, Narayanan K, and Makino S: Charaterization of coronavirus DI RNA packages. Coronaviruses and Arteriviruses In Advance and Experimental medicine and Biology, Plenum press New York 347-353:1998. 8. Kim KH, and Makino S. Expression of murine coronavirus gene 1 and 7 is sufficient for viral RNA synthesis: In Advance and Experimental medicine and Biology, Plenum press New York 479-484:1999. 9. Kim KH, and Makino S: Two murine coronavirus gene suffice for viral RNA synthesis. J.Virol 69:2313-2321:1995. 10. Narayanan K, Kim KH, Makino S: Charaterization of N protein self-association in coronavirus ribonucleotide complex. Virus Research 98:131~140931:2003. 11. Woo. K, M Joo, Narayanan K, Kim KH, Makino K: Murine coronavirus packaging signal confers packaging to non-viral RNA. J.Virol 71:824-827:1997 12. Zhang MY, Choudhry V, Xiao X, Dimitrov DS. Human monoclonal antibodies to the S glycoprotein and related protein as potential therapeutics for SARS: Curr Opin Mol Ther. Apr;7(2):151-6:2005. 13. Francesc Puig-Basagoiti, Mark Tilgner, Brrtt M et al: Triaryl Pyrazoline compound inhibits Flavivirus RNA replication. NTIMICROBIAL AGENTS AND CHEMOTHERAPY. Apr: 1320-1329:2006. 14. J Cinatl, B Morgenstern, G Bauer et al: Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. THE LANCET. June: 361:2003. 15. A.R. McCutcheon, T.E. Robert, E. Gibbons et al: Antiviral screening of British Columbian medicine plants. JOURNAL OF ETHNO-PHARMACOLOGY. Aug 101-110:1995. 16. Els Keyaerts, Leen Vijgen, Piet Maes et al: In vivo inhibition of severe acute respiratory syndrome coronavirus by chloroquine. BBRC Aug: 264-268:2004. 17. Adam M. A. and D Miller: Identification of a signal in a murine retrovirus that is sufficient for packaging of nonretrovirus RNA into virion. J.Virol 62:3802-3806:1988 18. Alford, R. L and J. W. Belmont: RNA secondary structure analysis of the packaging signal for Moloney murine leukemia virus. Virology 183: 611-619:1991. 19. Ahlquist, P. and E. G. Strauss: Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J. Virol 53:536-542:1985. 20. Baric, R. S. and G. W. Nelson: Interaction between coronavirus nucleocapsid protein and viral RNAs. J. Virol 62:4280-4287:1988. 21. Collins A. R. and R. L. Knobler: Monoclonal antibody to murine hepatitis virus-4 define the viral glycoprotein responsible for attachment and cell fusion. Virology 119:358-371:1982. 22. Compton, S. and D. B Rogers: In vitro replication of mouse hepatitis virus strain A59. J. Virol 61:1814-1820:1987. 출처: https://www.ndsl.kr/ndsl/search/detail/article/articleSearchResultDetail.do?cn=DIKO0010879168 |